ABOUT US

Luohe Chief Pharmaceutical Co., Ltd
Chief Pharma, Bring Good Health to Mankind
Ambition, Capability, Accountability, Responsibility
Founded in 2013, Chief Pharma is a high-tech enterprise, focusing on the research and development, production and sales of active pharmaceutical ingredients (APIs) and intermediates. We're featured with Rifamycin series products, specializing in the production of Rifamycin S, Rifampicin, Rifaximin, Rifabutin, Rifamycin Sodium and other products in a large scale. USDMFs and CEPs for Rifamycin series products have already been submitted, and registration in Europe, USA and other countries and regions has been completed.
Our headquarter is located in Luohe City, Henan Province, the hinterland of the central plains. We're committed to the research, development and production of APIs and intermediates in the field of anti-tuberculosis, anti-virus, cardiovascular and diabetes, as well as enzyme production technology in commercial production.
We always keep in mind that quality is the cornerstone of the survival, and that's why customers choose us. We strictly abide by the quality control, and offer reliable products to customers.
With ambition, capability, accountability and responsibility, we'll stay true to original aspiration, forge ahead and together build a bright future of Chief Pharma!

Company History
The company was registered and founded in Luohe, Henan
The factory was built in Yancheng District, Luohe City
Enzyme pilot plant was established
Enzyme fermentation plant was built
Enzymatic reaction plant, Enzymatic products refine plant were established
R&D, trial production of Rifamycin Sodium, Rifabutin, Rifaximin, Sodium Aminosalicylate, Rifamycin S were completed
The QC laboratory and QA system began to operate
Synthesis plants of Rifabutin, Rifaximin, Sodium Aminosalicylate, Rifamycin Sodium, Rifamycin S were completed
Site audit for Drug Manufacturing License from NMPA
Pilot production of Rifabutin, Rifaximin, Sodium Aminosalicylate, Rifamycin Sodium & Rifamycin S was completed
Process Validation for Rifabutin, Rifaximin, Sodium Aminosalicylate, Rifamycin Sodium, and Rifamycin S was started
Submission of USDMFs and CEPs for Rifabutin, Rifaximin, Rifamycin Sodium, Rifamycin S was began
USDMF submission for Rifamycin S was completed
WC for Rifaximin and Rifamycin Sodium were obtained
WC for Rifabutin was obtained
Production Environment
Corporate Culture
-

Target
Bring good health to mankind -

Responsibility
Keep the bottom line of safety, protect the rights and interests of employees and maintain the ecological environment -

Philosophy
Be reliable pharmaceutical enterprise, Be moral to mankind
Accreditation
Pollutant Discharge Permits Certificate
2022-07-04
National High-tech Enterprise Certificate
2021-10-28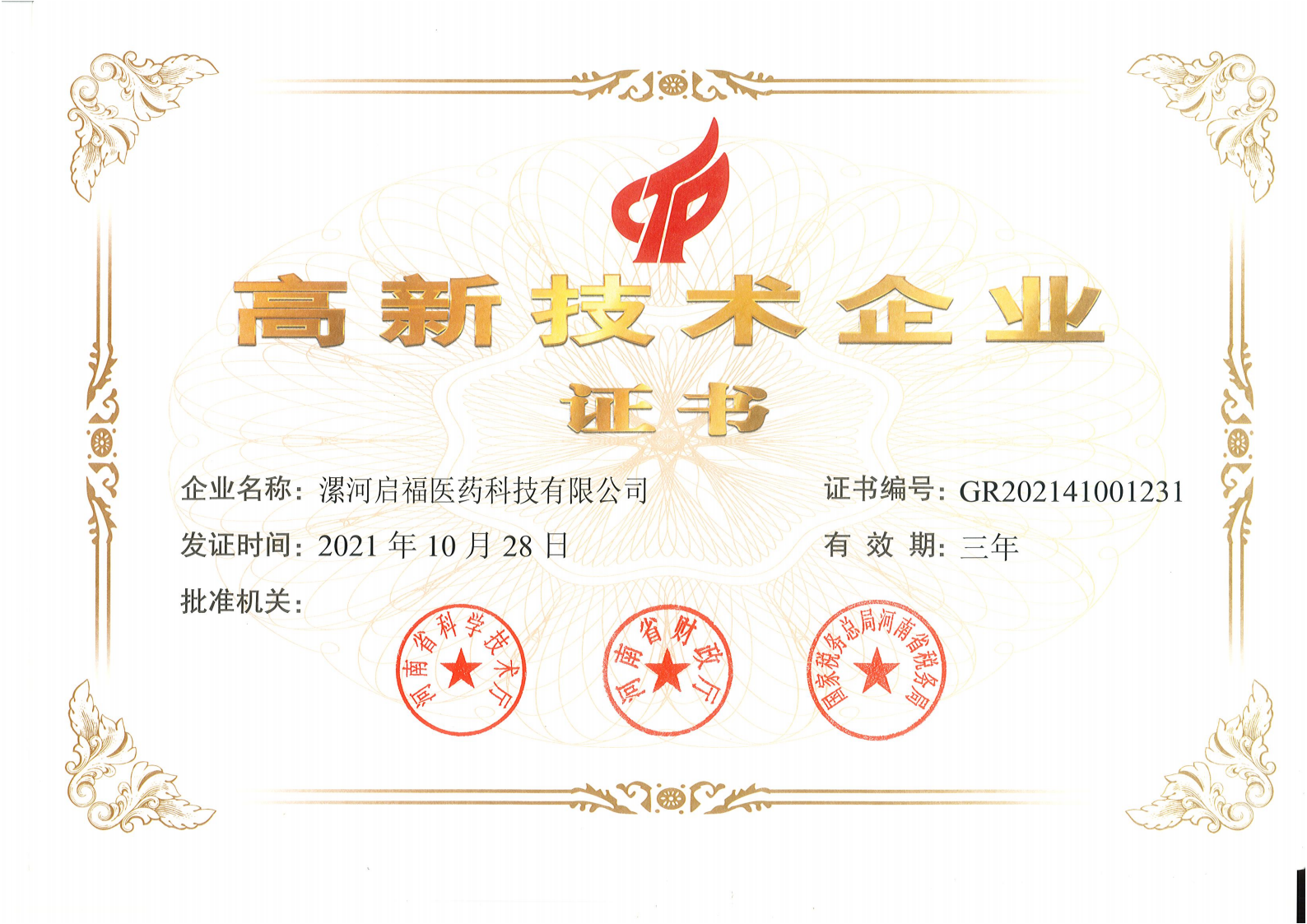
Drug Manufacturing License
2021-01-21

Copyright © 2021 Luohe Chief Pharmaceutical Co., Ltd 豫ICP备2021017587号-1









